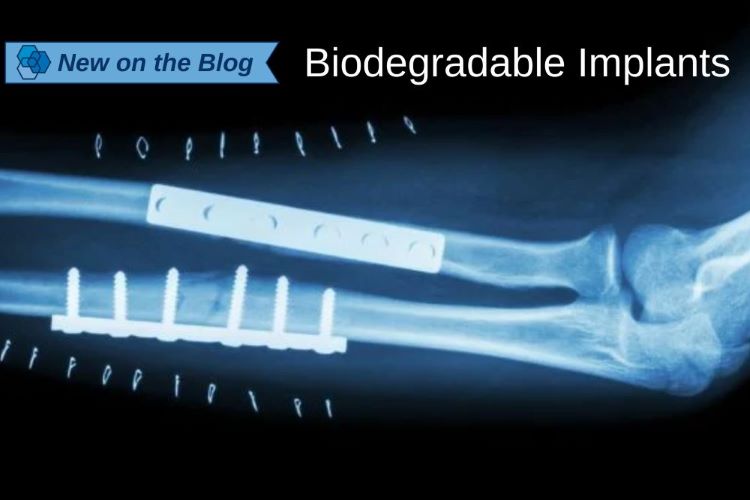
Navigating Liability Protections for Life Science Companies Under Blood and Tissue Shield Laws
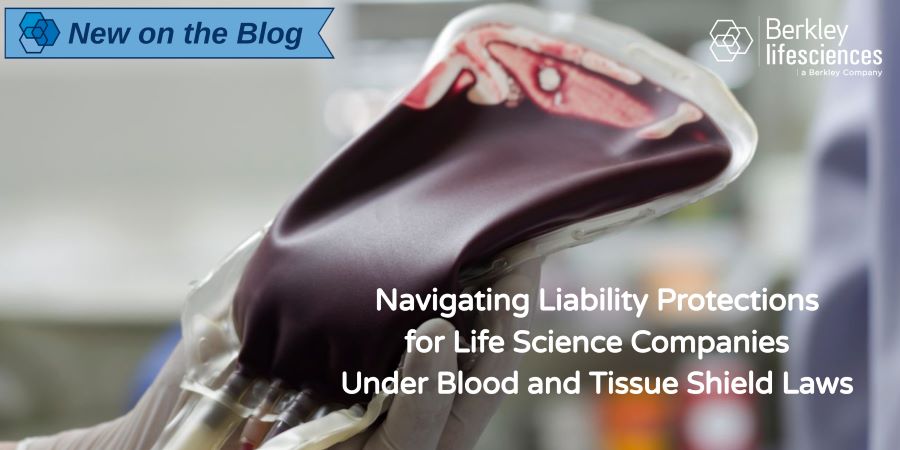
Beginning in the 1960s, medical facilities, blood banks, and other public providers of blood products began seeing limited statutory protections arise in most states through what has become known as “blood shield laws.”
Read More